Spine
We deliver the total spine experience, empowering our customers to keep the world moving. Together, we do more to challenge the standard, respond to clinical needs and pioneer new possibilities.
Our core technologies include biologic solutions, spinal implants, and surgical instruments for use in spine procedures. Our enabling technologies include digital health, guidance, and imaging for both cranial and spine procedures.

Balance ACS (BACS)
Balance ACS is a comprehensive platform that applies three-dimensional solutions across the entire clinical care continuum to help drive quality outcomes for spine patients. It provides tools – from surgical preauthorization and preoperative planning to 3D anatomical modeling and postoperative reporting – to support the perioperative process.
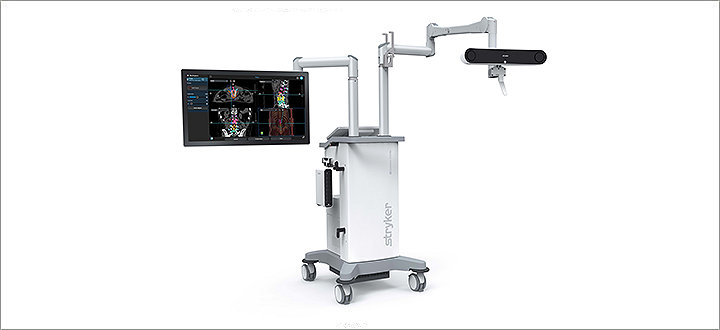
Spinal and cranial navigation
Featuring new optical tracking options, sophisticated software algorithms and instrumentation specifically designed for spine surgery, today’s Q Guidance System aims to deliver more surgical spine and cranial planning and navigation capability than ever before. And, when used in tandem with Airo TruCT, those abilities can be amplified even further. Their collective benefits are designed to help make healthcare better.

Airo TruCT Mobile CT Scanner
Airo TruCT is a 32-slice point-of-care CT scanner that combines self propelled mobility with the world's largest inner bore. Capable of capturing the entire spine with a single spin in as little as 43 seconds, Airo TruCT is designed to consider your patient's unique anatomy.1 It is designed to deliver customized radiation and potentially minimize radiation exposure.2
AMagineTechnology
AMagine is our proprietary approach to implant creation using additive manufacturing
Innovation at Stryker is not only present in our products, but also in our manufacturing and supply chain. At the AMagine Institute in Cork, Ireland, we are passionate about exploring, developing, and industrializing innovative technologies that enable the manufacturing of Stryker’s next generation of products – products that are designed to help make healthcare better. From groundbreaking design features to innovative materials and faster product availability, we’re exploring the latest 3D-printing technologies and digital systems to build state-of-the-art manufacturing and supply chains at scale.
Monterey AL
Interbody System
With a design for indirect decompression, a Tritanium structure to facilitate bone in-growth3 and a wide array of footprints, heights and lordotic options – our Monterey AL Interbody System aims to deliver on all fronts. Plus, our instrumentation is designed to support your surgical style whether it’s freehand, partially guided, or fully guided.

Serrato
This unique screw family features True-Tip technology, a dual lead thread pattern and 24 circumferential serrations on the distal tip of the screw. Its cortical cancellous thread pattern is a continuation of our clinically proven Xia design philosophy, and is built to maximize performance and purchase in both cortical and cancellous vertebral bone.
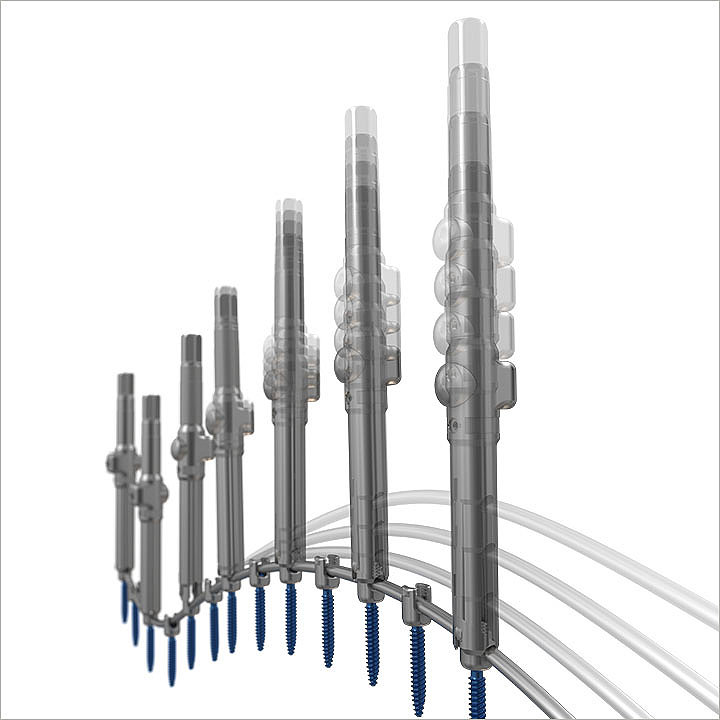
Everest Deformity
The Everest Deformity Spinal System features a top-loading pedicle screw in a variety of screw types and the ability to accommodate titanium and cobalt chrome rods of two different diameters. The revolutionary instrumentation is designed to address the most difficult correction maneuvers for complex spinal pathologies.
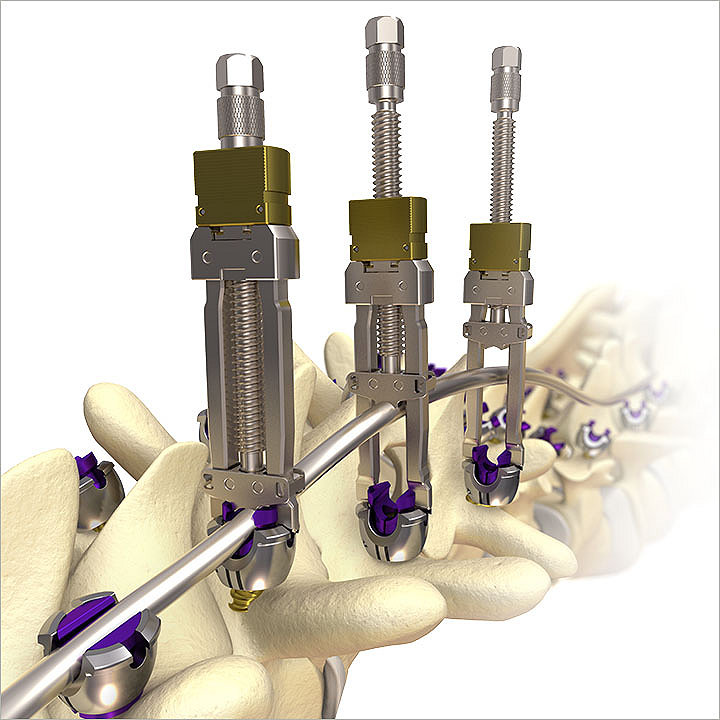
Mesa 2 Deformity
Mesa 2 screws are top-loading, low-profile, and feature Zero-Torque Technology. The streamlined instrumentation is designed for efficiency and ease of use. This system is poised to address difficult correction maneuvers for complex spinal pathologies.
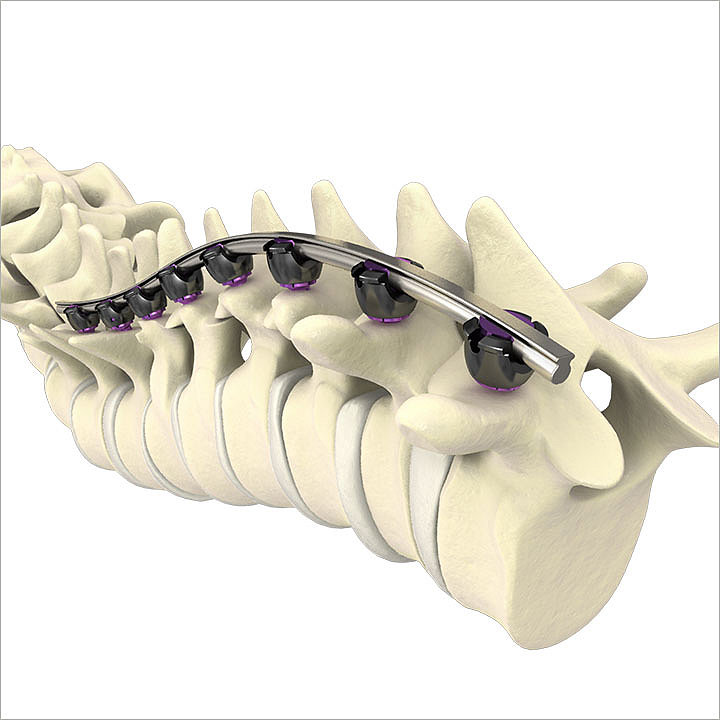
Rail 4D Technology
Rail 4D Technology incorporates a “beam–like” design providing rigidity to aid in the restoration of sagittal balance, while maintaining a low profile.

Niagara
Niagara is designed to help achieve desired placement while helping to minimize the need to reposition, so the focus is on the procedure; not the set-up. Engineered from a unique formulation of PEEK-infused carbon fiber, this retractor is strong and radiolucent, designed to help reduce the need for intra-operative, image-driven adjustments and allow for a clear view at level.

Cascadia Lateral 3D
The Cascadia Lateral 3D Interbody System includes a full range of implant sizes designed to accommodate the vertebral anatomy. The Cascadia Lateral 3D cages are built with Lamellar 3D Titanium Technology, incorporating 300-500μm longitudinal channels throughout the implant, which in conjunction with transverse windows, create an interconnected lattice design to allow for bony integration.4

Everest MI XT
The Everest Minimally Invasive (MI) XT Spinal System features Everest screw technology with metal extensions for minimally invasive rod delivery and reduction.

ProLift Expandable Spacer System
The ProLift Expandable Spacer System with Osseo-loc Surface Technology provides a micro invasive solution for TLIF and PLIF procedures.
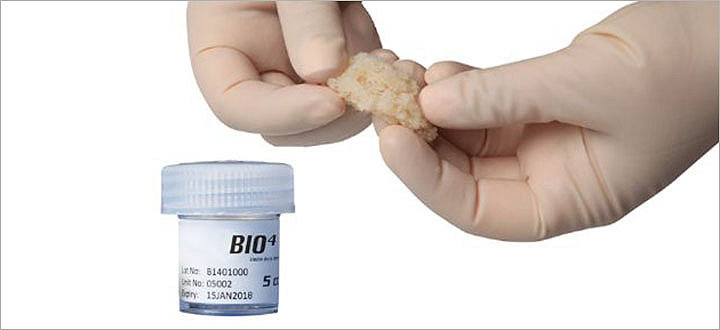
BIO4
BIO4 is a viable bone matrix containing endogenous bone forming cells (including mesenchymal stem cells, osteoprogenitor cells, and osteoblasts) as well as osteoinductive and angiogenic growth factors.
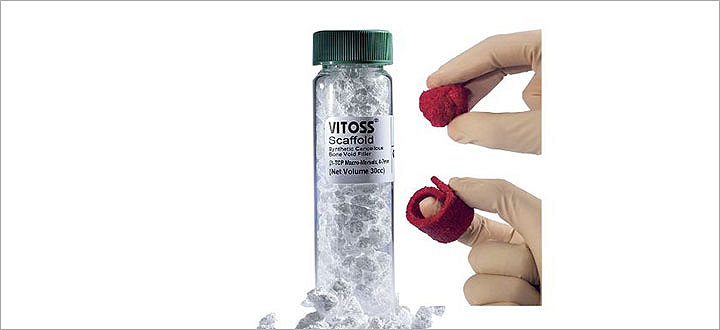
Vitoss
Vitoss is a synthetic bone graft with a unique interconnected, ultra-porous structure that resembles human cancellous bone. Available in three formulations (foam pack, foam strip or morsels and blocks), it possesses the same three bone healing components as iliac crest bone graft when it is combined with bone marrow aspirate – a scaffold (osteoconductive), cells (osteogenic) and signals (osteoinductive).5

Capri Cervical 3D Expandable
The Capri Cervical 3D Expandable Cage provides an innovative, 3D-printed solution for stabilization of the spine in cases of vertebral body resections resulting from trauma or tumor. The innovative Height/Angulation Driver allows for controlled in-situ height adjustment as well as the ability to intraoperatively adjust the angle of the endplates from 0-20 degrees.
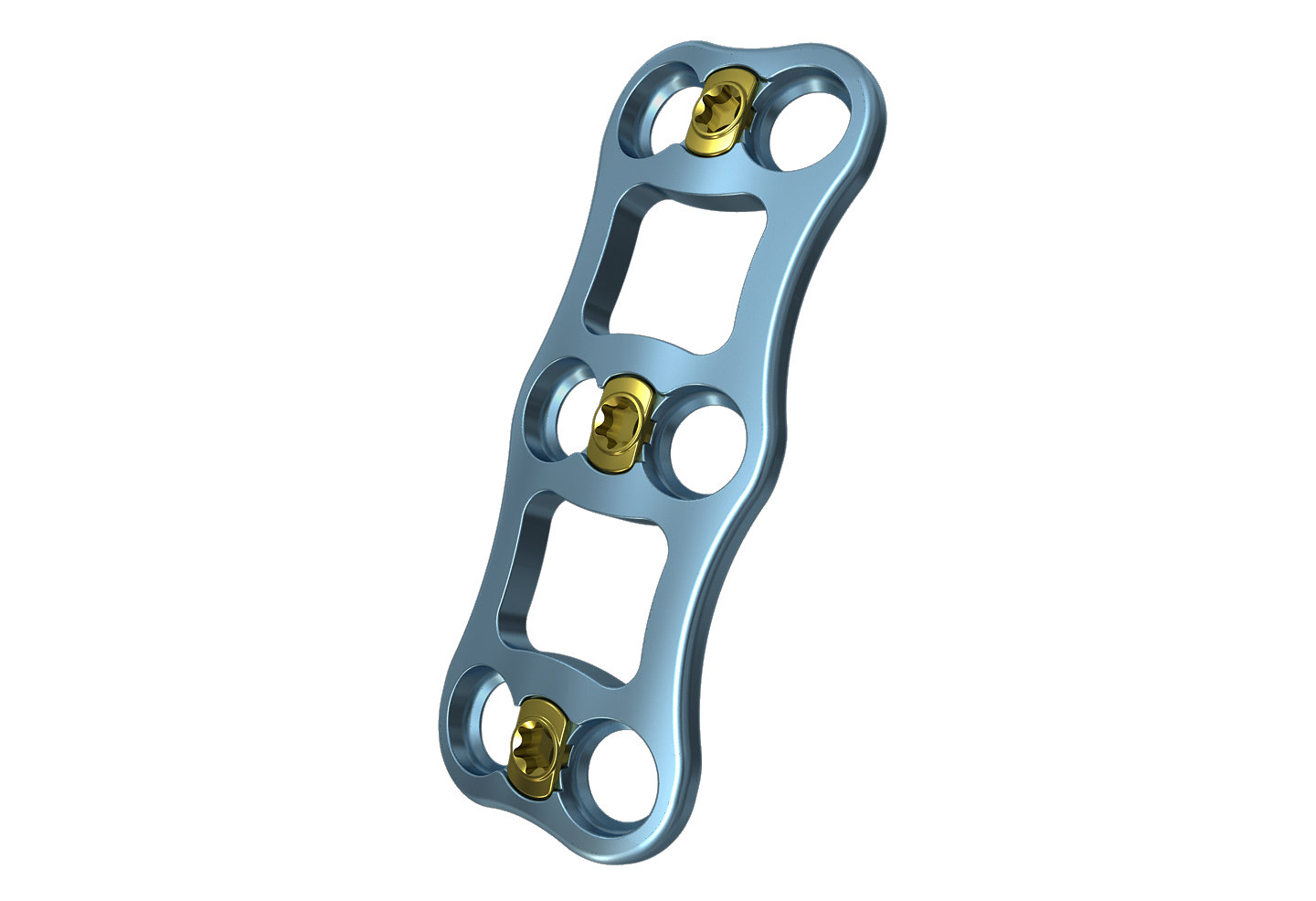
Ozark
The Ozark View Cervical Plate System features a per-level, integrated locking cover that provides visual and tactile confirmation of the final lock position. The View Plate features a wide window for increased graft visualization and includes a variety of implant sizes, screw dimensions, and surgical instruments

Tritanium C
Tritanium C is a hollow, cervical interbody cage that consists of a unique configuration of both solid and porous structures built using AMagine Technology, our proprietary approach to implant creation using additive manufacturing.

Yukon OCT
The Yukon OCT Spinal System features a top-loading polyaxial screw with high angulation and the ability to accommodate rods in two diameters. The system offers a comprehensive selection of polyaxial screws, hooks, rods, connectors, and occipital plates.
We are working hard to make healthcare better by partnering with our customers to help develop, implement and sustain patient-centric spine service lines.
We can help bring you closer to your clinical, operational and financial goals
Connect with us
Spine division
600 Hope Pkwy SE,
Leesburg, VA 20175
Looking for a product IFU?
Having trouble?
SpineIFURequest@stryker.com
Need a paper copy?
Call 855.236.0910
Spine Reprocessing Guide
References:
1. MI-19-0030 | Airo Design Verification Procedure
2. MI-48-0423 | Using the optional x-ray tube current modulation feature. As the scan translates, radiation is adjusted to the patient's specific anatomy based on the scout scan. This will come onscreen as this section is read. This feature is only available on Airo Software version 1.2 and higher.
3. PROJ 43909 | Tritanium technology claim support memo, Rev-1
4. K2M Cascadia TR-1220 Rev 1
5. Bellincampi, L., Clineff, T., Erbe, E. Osteoinductivity of Vitoss with Isologous Bone Marrow in Urist Rat Pouch Model. Society for Biomaterials. Tampa, FL. April 24-27, 2002 (Podium). GEN-WB-1_15462
QGS-WB-9_34766
